HOW OSMOSIS AFFECT ANIMAL CELL AND PLANT CELL
Osmosis and animal cell:
The figure illustrates an animal cell in pure water. The cytoplasm inside the cell is a fairly concentrated solution. The protein and many other substances dissolved in it are too large to get through the cell membrane. Water molecules, though, can get through.
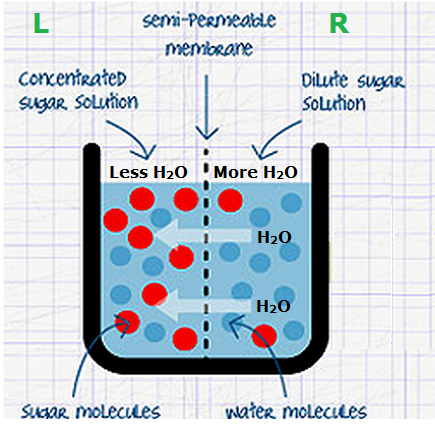
If you compare this situation with the figure osmosis, you will see that they are similar. The dilute solution in figure osmosis and the pure water in figure animal cell in pure water are each separated from the a concentrated solution by a permeable membrane is the cell membrane. Therefore, osmosis will occur.
Water molecule will diffuse from the dilute solution into the concentrated solution. What happens to the cell? As more and more water enters the cell, it swells. The cell membrane has to stretch as the cell gets bigger, until eventually the strain is too much, and the cell bursts.
An animal cell in a concentrated solution. If this solution is more concentrated than the cytoplasm, then water molecules will diffuse out of the cell. Look the picture of osmosis. to see why? As the water molecule go out through the cell membrane, the cytoplasm shrinks. The cell shrivel up.
Osmosis and plant cells-

Plant cell do no burst in pure water. The figure illustrates a plant cell wall. This is fully permeable, which means that it will let any molecules go through it.
Although it is not easy to see, a plant cell also has a cell surface membrane just like an animal cell. The plant cell in pure water will take in water by osmosis through its partially permeable cell in the same way as animal cell. As the water goes in, the cytoplasm and vacuole will swell.
However, the plant cell has a very strong cell wall around it. The cell wall is much stronger than the cell membrane and it stops the plant cell from bursting. The cytoplasm presses out against the cell wall, but the wall resists and presses and presses back on the contents.
A plant cell in this state rather like a blown up tyre- tight and firm. It is said to be turgid. The turgidity of its cells helps a plant that has no wood in it stay upright, and keeps the leaves firm. Plant cells are usually turgid.
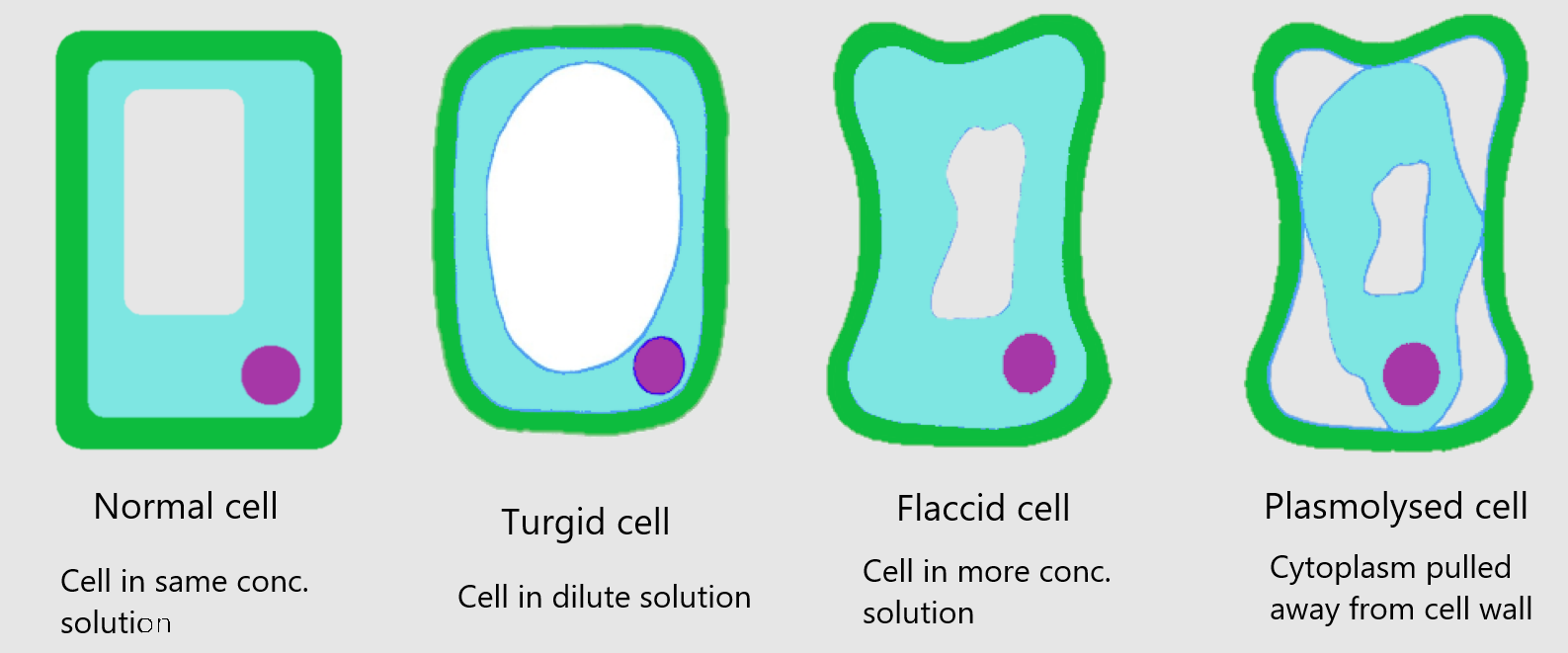
Figure 1 and figure 2 illustrate a plant cell in a concentrated solution. Like like the animal cell will lose water by osmosis. The cytoplasm shrinks, and stops pushing outwards on the cell wall. Like a tyre when some of the air has leaked out, the cell becomes floppy. It is said to be flaccid. If the cell in a plant become flaccid, the plant loses its firmness and begins to wilt.
If the solution is very concentrated, the a lot of water will diffuse out of the cell. The cytoplasm and vacuole go on shrinking. The cell wall though, is too stiff to be able to shrink much. As the cytoplasm shrinks further and further into the centre of the cell, the cell wall gets left behind. The cell membrane, surrounding the cytoplasm, tears away from the cell.
A cell like this is said to be plasmolysed. This does not normally happen because plant cells are not usually surrounded by very concentrated solutions. However, you can make cells become plasmolysed if you do osmosis. Plasmolysis usually kills a plant cell because the cell membrane is damaged as it tears away from the cell wall.